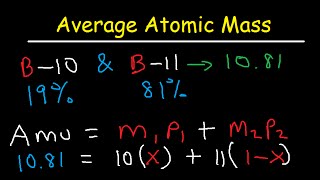
How To Calculate Average Atomic Mass With Percent Abundance
It provides equation / formula for do so. Using the weighted average (in form of atomic mass) and individual isotope masses to calculate percent abundances two isotopes.
how to calculate average atomic mass with percent abundance Indeed lately has been sought by users around us, perhaps one of you personally. Individuals are now accustomed to using the internet in gadgets to see video and image information for inspiration, and according to the title of the post I will discuss about How To Calculate Average Atomic Mass With Percent Abundance.
This video demonstrates how to calculate the average atomic mass (also called relative mass) for an element.
This chemistry video tutorial explains how to calculate the average atomic mass of an element given percent abundance each isotope. An example problem demonstrating how to calculate the mass of a specific isotope given natural abundance isotopes and one mas. This chemistry video tutorial shows you how to calculate the average atomic mass of 2 or 3 isotopes.
If you're looking for video and picture information related to the keyword How to calculate average atomic mass with percent abundance you have come to pay a visit to the right site. Our website gives you hints for seeing the highest quality video and image content, hunt and locate more informative video articles and images that fit your interests. How to calculate average atomic mass with percent abundance includes one of tens of thousands of video collections from several sources, especially Youtube, therefore we recommend this video for you to see. You can also bring about supporting this website by sharing videos and images that you enjoy on this blog on your social media accounts such as Facebook and Instagram or educate your closest friends share your experiences concerning the ease of access to downloads and the information that you get on this site. This site is for them to visit this site.



Calculations require you solve system o. A shorter video that shows the mathmatice needed to calculate percent abundances from average atomic masses. You need know each isotope and percent (%) abundance as well.
How to find the average atomic mass of an element. Multiply by its corresponding percentage, add. If you're given the mass of each isotope an element, and average atomic mass, you can calculate percent (%) abundance isotope.
So by making this site we just want to make it much easier for users to get info to be used as ideas. All content on this blog does not have an Admin, the Admin just wants to provide advice Info that matches alongside the key word Calculating The Mass For Isotopes With Natural Abundance may be helpful.
If you find this site useful to encourage us by sharing this blog post to your treasured social networking accounts like Facebook, Instagram etc or you could also bookmark this site page with the name Calculating The Mass For Isotopes With Natural Abundance using Ctrl + D for computers with operating systems Windows or Control + D for notebook devices with Mac OS. If you use a phone, you can even use the drawer menu of the browser you are using. Whether it's a Windows, Mac, iOS or Android operating platform, you will still have the ability to bookmark this site page.